Report Overview
Meat and Poultry Testing Highlights
Meat and Poultry Testing Market Size:
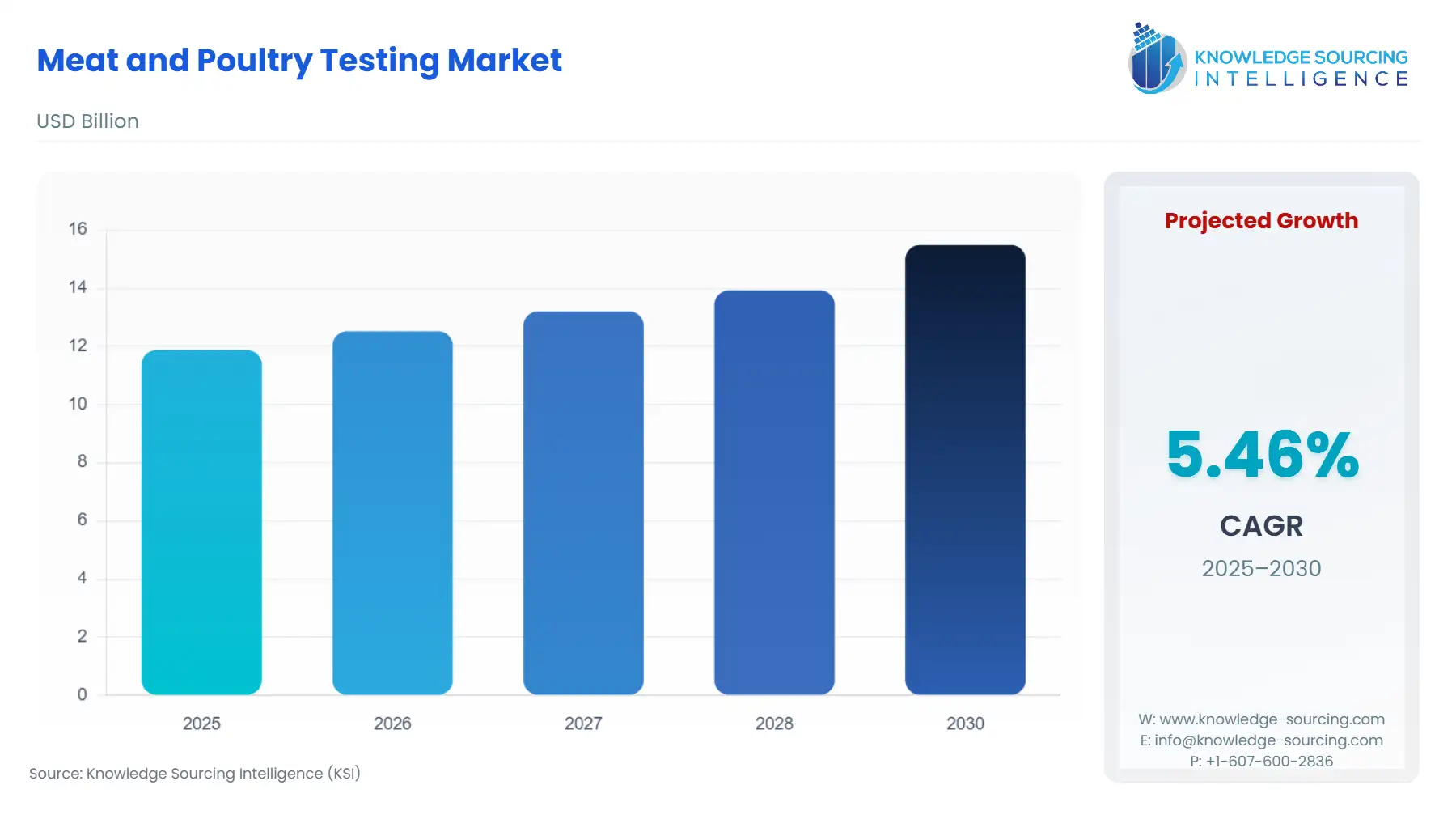
The Meat and Poultry Testing Market will expand from USD 11.873 billion in 2025 to USD 15.488 billion in 2030, with a 5.46% compound annual growth rate (CAGR).
The integrity of the global food supply chain rests heavily upon robust analytical testing, and the Meat and Poultry Testing Market is a critical node in this framework. This market encompasses the analytical services, consumables, and instrumentation used to detect pathogens, spoilage organisms, allergens, and chemical residues across the meat and poultry production lifecycle, from farm to finished product. Industry dynamics are fundamentally shaped by a dual imperative: satisfying evolving, highly technical regulatory mandates and managing escalating consumer anxiety over food safety events. Consequently, demand is rapidly concentrating on testing modalities that deliver speed, high throughput, and quantitative data, transforming testing from a simple compliance task into an essential, preemptive risk management tool for producers and processors.
Meat And Poultry Testing Market Analysis:
- Growth Drivers:
Regulatory overhaul, led by agencies like the FSIS and the European Food Safety Authority (EFSA), is the primary growth catalyst. New proposals establishing performance standards for pathogens, such as the requirement for quantitative data on Salmonella loads, compel meat and poultry processors to invest in sophisticated, real-time molecular diagnostics to monitor and immediately verify the efficacy of intervention steps. Simultaneously, pervasive media coverage of foodborne illness outbreaks significantly increases consumer awareness, compelling major retailers and food service operators to demand higher-frequency and more comprehensive testing protocols from their suppliers as a prerequisite for procurement. This shift effectively extends the regulatory burden throughout the commercial value chain, creating mandatory testing demand at every stage.
- Challenges and Opportunities:
The major constraint remains the lack of universally harmonized global testing standards, which fragments the market and forces manufacturers to utilize multiple testing methods to satisfy diverse export requirements, thereby increasing operational complexity and cost. However, this same environment creates significant opportunity for technology firms. The industry is urgently seeking rapid, on-site/near-line testing solutions to reduce the 48-72 hour hold times associated with traditional culture methods. The opportunity lies in deploying fully integrated, automated molecular systems that deliver accurate results within the processing shift, substantially enhancing supply chain efficiency and creating new demand for streamlined, enterprise-wide laboratory informatics solutions.
- Supply Chain Analysis:
The supply chain is vertically integrated, spanning the upstream provision of proprietary consumables and instrumentation to the downstream operation of commercial testing laboratories. Key production hubs for high-value molecular testing kits (e.g., PCR reagents, proprietary media) are concentrated in North America and Europe, leveraging specialized chemical and biotechnological expertise. Logistical complexity is centered on maintaining the cold chain for temperature-sensitive reagents and ensuring the rapid delivery of reference materials to decentralized processor laboratories globally. The market exhibits a heavy dependency on major laboratory service providers (e.g., Eurofins, Intertek) who manage high-volume, global testing requirements, while the technical component relies on manufacturers (e.g., Neogen, Bio-Rad) supplying the specialized, capital-intensive analytical instruments.
Meat And Poultry Testing Market Government Regulations:
Regulatory frameworks dictate the minimum frequency, type, and methodology of testing, acting as a direct and non-negotiable floor for market expansion. The global regulatory landscape is moving from presence/absence testing toward quantitative limits and precise serotyping for critical pathogens. This evolution mandates the continuous validation of new, advanced methods against established protocols (e.g., AOAC International and AFNOR validations). These standards not only enforce compliance but also accelerate technological adoption by making rapid molecular assays the de facto industry standard for verification and risk management.
|
Jurisdiction |
Key Regulation / Agency |
Market Impact Analysis |
|
United States |
FSIS (Food Safety and Inspection Service) / Proposed Salmonella Performance Standards |
The shift toward enforceable, quantitative product standards for Salmonella in poultry and ground meat compels processors to adopt quantitative PCR (qPCR) or similar methods, creating immediate demand for instrument and assay upgrades in internal and commercial labs. |
|
European Union |
Regulation (EC) No 178/2002 (General Food Law) / Food and Veterinary Office (FVO) Audits |
The "Farm-to-Fork" approach, supported by annual FVO audits and the Rapid Alert System for Food and Feed (RASFF), demands comprehensive testing for pathogens, residues (veterinary drugs, pesticides), and contaminants throughout the supply chain, ensuring sustained, broad-based testing demand. |
|
China |
General Administration of Customs (GACC) / Import Food Safety Requirements |
Stringent and frequently updated import testing and traceability requirements for meat and poultry products, often requiring specialized, GACC-accredited laboratory certification, directly drives demand for third-party lab services and compliance testing for all major global exporters. |
Meat And Poultry Testing Market Segment Analysis:
- By Testing: Pathogens
The Pathogens segment forms the commercial core of the market, driven by the non-negotiable public health and regulatory imperative to control foodborne illnesses caused by organisms like Salmonella, Campylobacter, and Shiga toxin-producing E. coli (STEC). Its requirement is highly inelastic and is currently surging due to two main forces. First, regulatory agencies are demanding greater resolution, shifting the focus from simple presence/absence screening to quantitative enumeration and serotyping. This transition immediately renders older, slower cultural methods insufficient, creating a massive replacement-cycle demand for molecular-based systems that can quantify the pathogen load and identify specific serotypes (e.g., Salmonella Typhimurium, Enteritidis) within a few hours. Second, processors and labs increasingly recognize that precise quantitative data, used in a Statistical Process Control (SPC) approach, allows them to immediately verify the effectiveness of sanitation and pathogen intervention steps, thereby driving demand for rapid assays that enable real-time operational decision-making, rather than post-mortem results.
- By Source: Chicken
The Chicken segment exhibits accelerated demand growth, disproportionate to other meat sources, primarily due to intense, targeted regulatory pressure and its high volume in global consumption. Regulatory bodies globally, and particularly the US FSIS, have historically focused modernization efforts on poultry, citing persistently high rates of Salmonella and Campylobacter contamination. This regulatory scrutiny translates into a direct, non-discretionary demand for testing services throughout the entire poultry production cycle, including breeder flocks, feed, processing environments, and final products. Specifically, the high-throughput, continuous nature of poultry processing necessitates the adoption of highly automated and rapid molecular testing systems to screen thousands of samples daily without interrupting line speed. The segment's growth is further compounded by its high volume in global trade, where exporters must satisfy multiple, often conflicting, national poultry testing standards, necessitating a dual investment in both high-speed screening and confirmatory reference testing capabilities.
Meat And Poultry Testing Market Geographical Analysis:
- US Market Analysis (North America):
The US market for meat and poultry testing is characterized by a high degree of integration between large-scale commercial processors and specialized contract laboratories. Its requirement is directly impacted by the FSIS’s modernization agenda, specifically its pivot toward controlling Salmonella through enforceable standards. This shift is fueling an immediate demand spike for quantitative assays and advanced serotyping capabilities, forcing major poultry and beef producers to deploy capital for molecular instrumentation upgrades. Furthermore, the mandatory E. coli O157:H7 testing requirement for beef trim and the classification of STEC strains as adulterants ensure sustained high-volume demand for real-time PCR testing in the beef sector, making fast turnaround time a major competitive differentiator for laboratories.
- Brazil Market Analysis (South America):
Brazil, as the world’s leading beef exporter and a major poultry producer, presents a testing market heavily governed by international trade dependencies. Domestic market growth is driven by the need to satisfy stringent import requirements from key trade partners, notably the EU, China, and the Middle East. The necessity is therefore highly focused on residue testing for veterinary drugs and hormones, alongside mandated pathogen screening. Major Brazilian meatpackers invest heavily in world-class in-house laboratories and third-party certifications (e.g., international ISO accreditation) to mitigate trade-related disruption, creating demand for high-end analytical chemistry instrumentation (e.g., LC-MS/MS) for chemical residue analysis and comprehensive traceability audits.
- Germany Market Analysis (Europe):
The German testing market, deeply embedded in the stringent European Union framework, is highly mature and technology-intensive. The requirement is less focused on basic pathogen screening (which is well-established) and more concentrated on specialized testing in two areas: antibiotic resistance and species authenticity (fraud). The EU's "One Health" strategy heavily scrutinizes the presence of antimicrobial-resistant bacteria, creating a niche but mandatory demand for specific genetic marker testing. Furthermore, a high-value consumer market drives demand for precise speciation testing (e.g., horse meat in beef) using DNA barcoding or PCR-based methods, transforming testing from a compliance necessity into a quality and trust assurance mechanism for major German retailers.
- UAE Market Analysis (Middle East & Africa):
The UAE market is a critical hub for imported meat and poultry, making its testing demand unique in its focus on Halal certification and stringent import control. Nearly all meat is imported, which necessitates rigorous pre-shipment and port-of-entry testing. The mandatory Halal certification process drives consistent demand for species authentication (Pork DNA detection) to prevent mislabeling, primarily using rapid PCR assays. Beyond this, a focus on securing a safe supply chain drives demanding surveillance testing for pathogens and residues at the entry point, creating high demand for contract laboratory services that can deliver internationally recognized ISO 17025 accredited results under short timelines for customs clearance.
- China Market Analysis (Asia-Pacific):
China's meat and poultry testing market is expanding rapidly, fueled by growing domestic consumption and a heightened sensitivity to food safety events. The sheer scale of the domestic supply chain and a zero-tolerance policy for positive pathogen findings in imported products drives this demand. The market creates substantial demand for high-throughput, affordable testing kits and automated equipment to manage the high volume of samples from domestic production. For imported products, specific GACC mandates for pathogens, heavy metals, and chemical residues necessitate that international exporters utilize verified, high-volume testing service providers who can demonstrate full compliance with complex, localized testing specifications.
Meat And Poultry Testing Market Competitive Environment and Analysis:
The Meat and Poultry Testing Market is characterized by intense competition between instrument/assay manufacturers and large, globally distributed laboratory service providers. Consolidation remains a key strategic theme as companies seek to offer end-to-end solutions, integrating rapid on-site screening with confirmatory reference laboratory analysis. Competitive advantage is derived not only from assay performance metrics (speed, specificity, quantification limits) but also from regulatory recognition (e.g., AOAC/AFNOR validation) and a robust global logistical footprint to support international trade.
- Eurofins
Eurofins operates as a highly diversified, global network of over 1,000 laboratories, strategically positioning it as a dominant reference laboratory service provider. Its competitive edge is rooted in its extensive geographic coverage and its ability to offer a comprehensive range of testing—from routine pathogen screens to complex chemical residue analysis (e.g., LC-MS/MS for veterinary drugs) and advanced authenticity testing. Eurofins’ strategy focuses on continuous, targeted acquisitions to expand its local market presence and technical portfolio, enabling it to service complex multinational food processors and retailers who require a single partner for global compliance testing and certification across multiple product lines and jurisdictions.
- Neogen
Neogen focuses on the upstream provision of proprietary consumables and rapid diagnostic kits, specializing in food safety, animal safety, and genomics. The company’s core strategic positioning targets the in-house testing market for meat and poultry processors. Its flagship molecular detection system, coupled with quantitative Salmonella and Listeria assays, directly addresses the industry's imperative to reduce processing hold times by providing actionable results within a single shift. Neogen’s strategy is heavily centered on developing user-friendly, validated platforms that enable non-specialist personnel in a processing plant to conduct screening and internal quality control, shifting demand away from external contract labs for primary screening.
- Bio-Rad Laboratories Inc.
Bio-Rad maintains a strong competitive position in the molecular diagnostics segment, particularly with its iQ-Check line of real-time PCR (qPCR) pathogen detection kits and its Droplet Digital PCR (ddPCR) technology. The company leverages its deep expertise in life science research to bring high-sensitivity, high-precision tools into the food safety testing sphere. Bio-Rad’s strategic focus is on solving the most technically arduous food safety challenges, such as Shiga toxin-producing E. coli (STEC) serotyping and the detection of low-level contamination. Its validated PCR kits for key meat and poultry pathogens are critical tools for commercial and government reference laboratories that require the highest standard of non-ambiguous, certifiable results.
Meat And Poultry Testing Market Developments:
- July 2025: Bio-Rad Laboratories Inc. expanded its high-sensitivity Droplet Digital PCR (ddPCR) offering through the acquisition of Stilla Technologies. The move enhances Bio-Rad's molecular portfolio, providing a more comprehensive suite of tools for the highly sensitive, absolute quantification of pathogens and contaminants necessary for increasingly rigorous regulatory environments.
- March 2025: Bio-Rad Laboratories Inc. launched the XP-Design Assay Salmonella Serotyping Solution. This product enables the rapid and precise characterization of Salmonella serotypes in food and environmental samples using PCR, directly addressing the growing demand from regulatory bodies for enhanced serotype-specific surveillance in the meat and poultry supply chain.
- January 2025: Neogen Corporation launched the Neogen Molecular Detection Assay 2 – Quantitative Salmonella (MDA2QSAL96). This release provides a quantitative molecular method for poultry rinses and ground meat, positioning the company to capitalize on the FSIS's push for quantitative Salmonella performance standards by providing processors with an effective verification tool.
Meat And Poultry Testing Market Segmentation:
By Testing
- Pathogens
- Bacteria
- Allergens
- Contamination
- Nutrition
- Rancidity Spoilage
By Source
- Beef
- Pork
- Sausages
- Chicken
- Eggs and products
- Others
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- United Kingdom
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- India
- Japan
- South Korea
- Indonesia
- Thailand
- Others