Increasing Innovations in the Antipsychotic drugs Industry

Antipsychotic medications are used to treat bipolar disorder and schizophrenia as well as other forms of psychosis. Psychosis is a severe indicator of mental illness that impairs reality and comprehension. Community-based initiatives that used to regularly bring people together have been severely disrupted; according to reports from several countries, clubs, organizations, and community-based initiatives have not been able to meet for months. There is an expectation that psychiatric diseases are going to have more patients than they do now. Moreover, illnesses masquerading as psychosis-schizophrenia, bipolar disorder as well as depression are also a part of this.
Further, the need for in-person mental health services has reportedly decreased due to fear of infection, especially in the elderly. Moreover, many organizations have been forced to adopt telehealth systems whereby individuals can be given advice through teleconferencing or websites even though the quality of such services greatly differs. Consequently, telemedicine permits physicians to give instructions concerning prescription and nonprescription drug administration and relevant nonprescription medications. It is anticipated that this will quicken market expansion in the future. These medications are commonly overprescribed, which can have detrimental effects and result in addiction. People in affluent countries such as North America are affected by this. This has led to tight government regulation of these medications, which has hampered their commercial growth. Furthermore, the introduction of pharmaceuticals in more contemporary forms, like long-acting injectables, will support the market's growth during the forecast period.
At the same time, a switch to organic products can be observed as of late. Such products include anti-ageing creams containing no chemicals but containing natural vitamins such as the E-title of the product. The increase in product applications in cosmeceuticals, providing perfect medical substitutes for usual medications, as well as the buoying consumption of augmented feed among animals, are the driving forces behind the market expansion. An ageing population, increased sales of functional foods and beverages, growing consumer purchasing power along with emergence of antipsychotic drugs supplements available in either online or offline stores are some of the other things that are helping in the expansion of this market.
Global Production Trends
The 1950s saw the emergence of first-generation antipsychotic drugs that belong to classic antipsychotics such as antipsychotic drugs. These drugs were suspected to cause visual impairment or drowsiness, which could be fatal if not managed well. Moreover, given the availability of newer, more potent medicines with fewer negative effects, it is projected that the development of these commodities will decrease in number in the time ahead.
Further, they are called atypical antipsychotic drugs and are used for treating schizophrenia instead of classical antipsychotic drugs. They include: clozapine, risperidone, sertindole, asenapine, olanzapine, paliperidone, and quetiapine.
Supplement Use Case
Schizophrenia is a dangerous mental illness that has a high prevalence worldwide. The disorder’s main features include alteration in thought processes perception, self-awareness distortion called "delusions" behavioural disorganization known as "hallucinations" among others key drivers to market development between now and the year 2026 are; an increase in elderly people who are more prone to catch late-onset types of this disease due to age advanced years. Furthermore, the increasing frequency rate of related disorders as well as schizophrenia itself has also contributed to its growth.
Further, regarding the creation of these drugs, several research and development projects have been undertaken. The purpose of these R&D efforts was to improve the effectiveness and safety of these medications. The greater adoption of these medications in important countries as a result of increased awareness led to a notable increase in market growth in those countries. Throughout the years, the R&D culture for these drugs has expanded in spite of the strict regulations surrounding drug approval, adoption, and reimbursement. It is anticipated that these medications' clinical trials will significantly advance the drug development process. For instance, the FDA approved Rykindo® (risperidone) as an extended-release injectable suspension in January 2023. Patients with bipolar I disorder are prescribed it as a biweekly treatment for schizophrenia and as monotherapy or adjunctive therapy to lithium or valproate. The atypical antipsychotic Rykindo® is made by Luye Pharmaceutical. Risperidone, the drug's active component, is injected intramuscularly using long-acting, extended-release microsphere technology. For this population, the approval of the biweekly extended-release injectable has subsequently brought about a promising therapeutic option.
Market Dynamics and Drivers
The growth of psychotic disorders results in an increasing consumption rate of such medicines around the world. For instance, it is projected that by the year 2030, mental illnesses will account for 6.0 trillion United States dollars of the global economy according to a 2022 article from World Health Organization (WHO). It is forecasted that more people will get to understand how serious mental illnesses are.
Further, the Centers for Disease Control and Prevention released data from a study for 2023 which showed that more than half of American adults would be suffering from mental illnesses. However, there has been an increase in Bipolar Disorder, Schizophrenia prevalence rates over the last few years. As a result, numerous pharmaceutical enterprises are already conducting research concerning such medicines leading to the introduction of fresh products within the predicted period
Figure 1: State of Mental Health in America, in Percentage, 2022
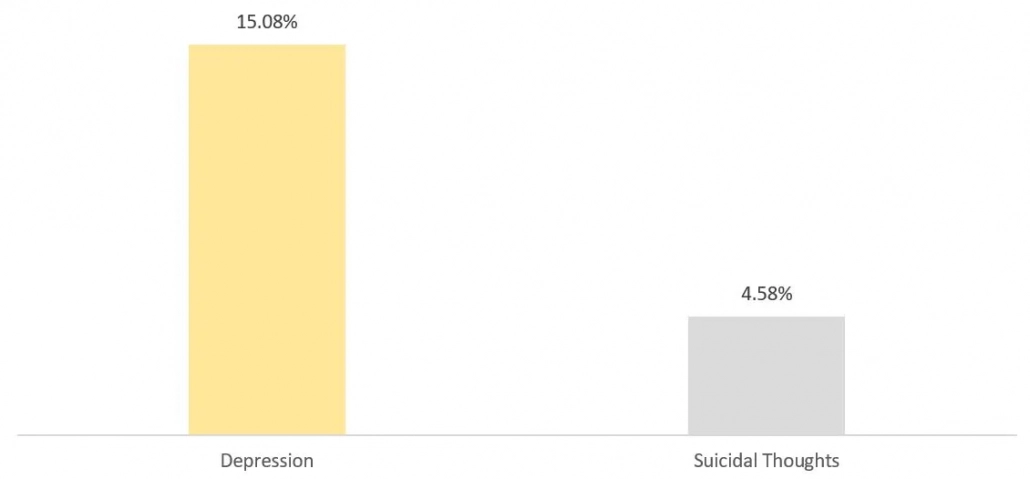 Source: Mental Health America
Key Developments
Source: Mental Health America
Key Developments
 Source: Mental Health America
Key Developments
Source: Mental Health America
Key Developments
- In April 2023, Teva Pharmaceuticals, an American subsidiary of Teva Pharmaceutical Industries Ltd., and MedinCell announced that UZEDY, an extended-release injectable suspension containing risperidone, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of schizophrenia in adults. UZEDY is the first long-acting, subcutaneous risperidone formulation that makes use of MedinCell-exclusive SteadyTeqTM, a copolymer technology that regulates risperidone's steady release. After a single dosage, therapeutic blood concentrations are attained in 6–24 hours.
- In January 2023, the Food and Drug Administration approved Rykindo for the treatment of schizophrenia and bipolar 1 disorder, according to Luye Pharma Group.
Get in Touch
Interested in this topic? Contact our analysts for more details.
Latest Blogs

Solar Control Window Films Market expected to reach USD 1,224.951 million by 2030
RecentlyTop Companies Leading the Silicon-Based Capacitor Revolution
Recently
The Role of Chemical Blowing Agents in Sustainable Foaming Solutions
Recently
Top 10 Emerging Beverages Set to Dominate the Market in the Coming Years
Recently